
Insights+: The US FDA New Drug Approvals in October 2023
Shots:
-
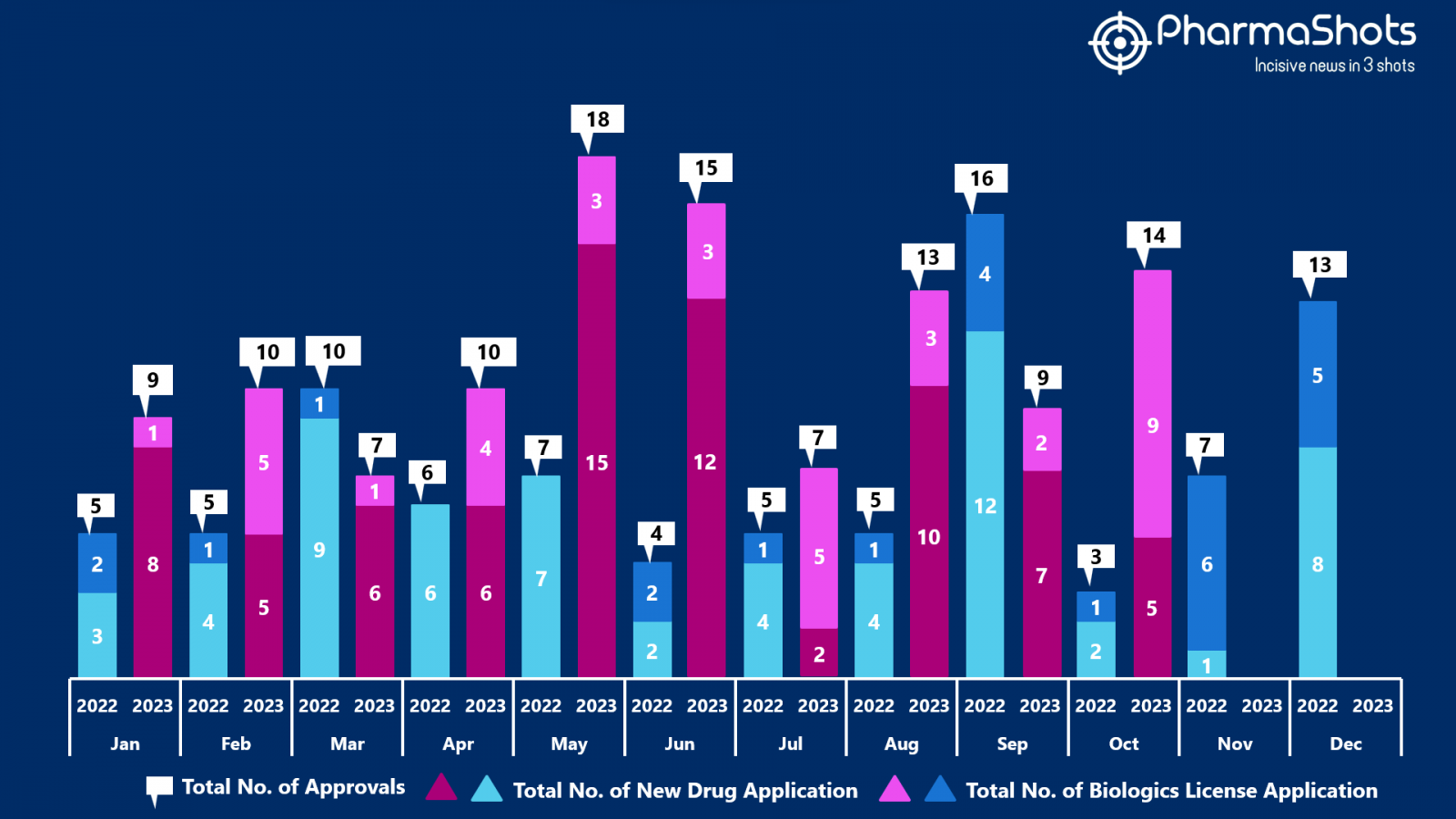
The US FDA approved 5 NDAs & 9 BLAs in October 2023, leading to treatments for patients and advances in the healthcare industry. The CDER and CBER approved 98 novel products in 2023
-
In October 2023, the major highlights drugs were Velsipity (Etrasimod) approved for Ulcerative Colitis, and Bimzelx (bimekizumab) for the Treatment of Adults with Moderate to Severe Plaque Psoriasis
-
PharmaShots has compiled a list of a total of 14 new drugs approved by the US FDA in October 2023
Novartis’ Cosentyx (secukinumab) Receives the US FDA’s Approval as First Intravenous Formulation for Rheumatic Diseases
Cosentyx
Active ingredient: Secukinumab Approved: Oct 6, 2023
Company: Novartis Disease: Rheumatic Diseases
-
The US FDA has approved an IV formulation of Cosentyx (secukinumab) for the treatment of adults with PsA, AS, and non-radiographic axial spondylarthritis (nr-axSpA). The new IV administration option will be available in Q4’23
-
The therapy offers HCPs choice and flexibility to tailor treatment to their patient's unique needs & IV formulation of Cosentyx provides patients a monthly 30-minute, weight-based dosing option that does not require pre-medication and lab monitoring
-
Cosentyx is a fully human biologic that specifically targets and blocks interleukin-17A (IL-17A). The therapy was approved in 100+ countries & was recently approved for JIA and hidradenitis suppurativa in the EU
Pfizer’s Braftovi (encorafenib) + Mektovi (binimetinib) Receives the US FDA’s Approval for BRAF V600E-Mutant Metastatic Non-Small Cell Lung Cancer
Braftovi & Mektovi
Active ingredient: Encorafenib & Binimetinib Approved: Oct 11, 2023
Company: Pfizer Disease: Cancer
-
The US FDA has approved Braftovi + Mektovi for metastatic NSCLC with a BRAF V600E mutation. The approval was based on the P-II trial (PHAROS) of Braftovi + Mektovi in treatment-naïve & prior treated patients with BRAF V600E-mutant metastatic NSCLC
-
The study met its major efficacy outcome measures of ORR & DoR. For treatment-naïve & previously treated patients, ORR (75% & 46%), and patients responded for 12mos. (59% & 33%), m-DoR was not estimable & 16.7mos. The results were presented at ASCO 2023 & published in the JCO
-
Patients experienced an adverse reaction resulting in permanent discontinuation of Mektovi & Braftovi (17% & 16%) & serious adverse reactions (38%). Braftovi + Mektovi was approved in the US for unresectable or metastatic melanoma with a BRAF V600E or V600K mutation
Pfizer’s Etrasimod Receives the US FDA’s Approval for Adults with Moderately to Severely Active Ulcerative Colitis
Velsipity
Active ingredient: Etrasimod Approved: Oct 12, 2023
Company: Pfizer Disease: Ulcerative Colitis
-
The approval was based on the P-III registrational program (ELEVATE UC 52 & 12) evaluating etrasimod (2mg, qd) vs PBO in UC patients who had previously failed or were intolerant to one conventional, biologic, or JAK inhibitor therapy
-
Both studies meet all primary & key secondary efficacy EPs with a favorable safety profile. In the (ELEVATE UC 52) study, clinical remission was 27.0% vs 7.0% at 12wk. & 32.0% vs 7.0% at 52wk. while 26.0% vs 15.0% in (ELEVATE UC 12) study
-
All key secondary efficacy EP were met at 12wk. incl. endoscopic improvement and mucosal healing. The safety of etrasimod was consistent with previous studies. The EMA has accepted the MAA for etrasimod with an expected decision at the beginning of 2024
Opdivo
Active ingredient: nivolumab Approved: Oct 13, 2023
Company: BMS Disease: Melanoma
-
The US FDA has approved Opdivo for adjuvant treatment of adult & pediatric patients aged ≥12yrs. with completely resected stage IIB or IIC melanoma. The approval was based on the P-III trial (CheckMate -76K) evaluating Opdivo (480mg, IV, q4w) vs PBO
-
The results showed a 58% reduction in risk of recurrence, new primary melanoma, or death, RFS rate (89% vs 79%) at 1yr. In a pre-specified exploratory subgroup analysis, the RFS unstratified HR in patients with stage IIB & IIC melanoma (0.34 & 0.51), 1 yr. RFS rates 93% vs 84% in stage IIB; 84% vs 72% in stage IIC
-
The results will be presented at the SMR annual meeting. Opdivo was approved in the US for the adjuvant treatment of adult & pediatric patients aged ≥12yrs. with melanoma with involvement of lymph nodes or metastatic disease
Zilbrysq
Active ingredient: zilucoplan Approved: Oct 17, 2023
Company: UCB Disease: Myasthenia Gravis
-
The US FDA has approved Zilbrysq for gMG in adult patients who are AChR Ab+. The approval was based on the P-III study (RAISE) results of Zilbrysq (SC, 0.3mg/kg) vs PBO, published in The Lancet Neurology
-
The study demonstrated that Zilbrysq provides rapid, consistent & significant benefits in different patient & clinician-reported outcomes at 12wk., improvements in MG-specific efficacy outcomes & significant improvement from baseline for MG-ADL & QMG total score while other 2EPs incl. the proportion of patients with improvements of 3 & 5 points in the MG-ADL & QMG total score at 12wk. without rescue therapy
-
Zilucoplan was approved in Japan for gMG in adult patients who inadequately respond to steroids or other immunosuppressants & is under review by TGA & Health Canada
UCB’ Bimzelx (bimekizumab) Receives the US FDA’s Approval for the Treatment of Adults with Moderate to Severe Plaque Psoriasis
Bimzelx
Active ingredient: bimekizumab Approved: Oct 17, 2023
Company: UCB Disease: Plaque Psoriasis
-
The approval was based on 3 P-III trials (BE READY, BE VIVID and BE SURE) evaluating bimekizumab in 1480 adults which showed that all studies met their co-1EPs & all ranked 2EPs
-
Patients treated with bimekizumab achieved superior levels of skin clearance at 16wk. vs ustekinumab, PBO & adalimumab as measured by PASI 90 & an IGA response of clear or almost clear skin (IGA 0/1). The 2EPs incl. PASI 75 at 4wk. & PASI 100 (complete skin clearance) at 16wk.
-
8 out of 10 patients who received bimekizumab (320mg, q4w) achieved PASI 90 & IGA 0/1 at 16wk. 6 out of 10 patients achieved PASI 100, clinical responses were rapid with more than 7 out of 10 patients achieving PASI 75 at 4wk. following 1 dose (320mg), clinical responses at 16wk. (PASI 90 & 100) were maintained for ~1yr.
Celltrion’s Zymfentra (Infliximab-Dyyb) Receives the US FDA’s Approval for the Treatment of Inflammatory Bowel Disease
Zymfentra
Active ingredient: Infliximab-Dyyb Approved: Oct 20, 2023
Company: Celltrion Disease: Inflammatory Bowel Disease
-
The approval was based on the P-III clinical trials (LIBERTY-UC) & (LIBERTY-CD) evaluating the safety & efficacy of Zymfentra vs PBO as maintenance therapy in patients (n=438 & 343) with UC & CD following treatment with an infliximab
-
The results from both studies depicted a greater clinical remission at 54wks. with Zymfentra (43.2% & 62.3%) vs PBO (20.8% & 32.1%) whereas the endoscopic response rate evaluated in the (LIBERTY-CD) trial was also seen to be greater in Zymfentra (51.1%) vs PBO (17.9%). The safety for both studies was similar to PBO during the maintenance period
-
Zymfentra is a subcutaneous version of Celltrion’s infliximab biosimilar which blocks the action of TNF-alpha
Servier’s Tibsovo Receives the US FDA’s Approval for the Treatment of Myelodysplastic Syndromes (MDS)
Tibsovo
Active ingredient: Ivosidenib Approved: Oct 24, 2023
Company: Servier Disease: Myelodysplastic Syndromes
-
The approval was based on the P-I clinical trial evaluating Tibsovo in patients (n=18) with IDH1-mutated r/r MDS. MDS is the 5th approved indication for Tibsovo, a precision medicine that targets the IDH1 mutation
-
The results depicted a CR rate of 38.9%, ORR of 83.3% & a median time to CR of 1.9mos. whereas at data cutoff the median duration of CR was not reached & mOS was 35.7mos. Moreover, 6/9 patients who were RBC transfusion-dependent became independent during ≥56-day post-baseline period
-
Tibsovo received BTD & Priority Review from the US FDA for IDH1-mut r/r MDS. Additionally, the US FDA also approved Abbott’s RealTime IDH1 Assay as a companion diagnostic device to select patients for Tibsovo
Eli Lilly’ Omvoh (mirikizumab-mrkz) Receives the US FDA’s Approval for Adults with Moderately to Severely Active Ulcerative Colitis
Omvoh
Active ingredient: mirikizumab-mrkz) Approved: Oct 26, 2023
Company: Eli Lilly Disease: Ulcerative Colitis
-
The approval was based on the (LUCENT) program incl. 2 P-III trials i.e., one 12wk. induction study (UC-1) and one 40wk. maintenance study (UC-2) for 52wks.
-
In (UC-1 & 2) trials, clinical remission (24% vs 15% & 51% vs 27%), endoscopic improvement (34% vs 21% & 58% vs 30%); histologic-endoscopic mucosal improvement (25% vs 14% & 43% vs 22%), clinical response (65% vs 43%) in (UC 1), (51% vs 27%) of all patients and 45% vs 15% who failed prior treatment with a biologic or JAKi achieved clinical remission at 1yr., improvement of symptoms i.e., rectal bleeding & stool frequency were seen as early as three weeks
-
In (UC2), steroid-free clinical remission (50% vs 27%) at 1yr. who achieved clinical response at 12wks. In a post-hoc analysis, 99% were steroid-free, and 66% vs 40% maintained clinical remission. The product will be available in the US in the coming weeks
Genentech’s Vabysmo (faricimab-svoa) Receives the US FDA’s Approval for the Treatment of Retinal Vein Occlusion
Vabysmo
Active ingredient: faricimab-svoa Approved: Oct 26, 2023
Company: Genentech Disease: Retinal Vein Occlusion
-
The approval was based on the P-III (BALATON) in 553 patients with branch retinal vein occlusion and (COMINO) studies in 729 patients with central retinal or hemiretinal vein occlusion evaluating Vabysmo vs aflibercept
-
The results showed that patients treated with the monthly treatment with Vabysmo achieved early and sustained improvement in vision in patients with branch and central RVO & met the 1EPs of non-inferior visual acuity gains at 24wks. & also showed that patients achieved rapid and robust drying of retinal fluid
-
The therapy was well tolerated in both studies & the safety profile was consistent with prior results. The therapy was approved in 80+ countries globally for wet AMD and DME
Agamree
Active ingredient: vamorolone Approved: Oct 26, 2023
Company: Santhera Disease: Duchenne Muscular Dystrophy
-
The approval was granted based on the data from the P-IIb (VISION-DMD) clinical trial evaluating the safety & efficacy of Agamree (2-6mg/kg/day) vs SoC corticosteroids in patients with DMD for up to 48mos. across 32 clinical trials in 11 countries
-
As per the agreement between Santhera & Catalyst Pharmaceuticals, following the approval, Santhera will receive $36M ($10M as milestone payments + $26M to cover contracted third-party milestone obligations) from Catalyst. Additionally, the US marketing authorization of Agamree will be transferred to Catalyst which expects to launch Agamree across the US in Q1’24
-
Santhera also received a positive opinion from the CHMP for which the EMA approval is expected by YE 2023
Coherus and Junshi Biosciences Received the US FDA’s Approval for Loqtorzi (toripalimab-tpzi) to Treat Nasopharyngeal Carcinoma (NPC)
Loqtorzi
Active ingredient: toripalimab-tpzi Approved: Oct 27, 2023
Company: Coherus and Junshi Biosciences Disease: Nasopharyngeal Carcinoma
-
The US FDA approval for Loqtorzi in combination with CT for 1L treatment and as monotherapy was based on P-III (JUPITER-02; n=289) & P-II (POLARIS-02) clinical trials on adults with metastatic or recurrent locally advanced NPC
-
The results of P-III (JUPITER-02) demonstrated improved PFS, reducing the risk of progression of the disease by 48% & OS with a 37% death reduction rate vs CT alone whereas mOS was not reached with the combination therapy vs 33.7mos. in CT alone. The P-II (LOQTORZI) results showed an ORR of 20.5%, DCR of 40% & median OS of 17.4mos.
-
Loqtorzi is an anti-PD-1 mAb that blocks PD-1 ligands PD-L1 & PD-L2 thereby activating the immune system and leading to the killing of tumors
Amgen Receives the US FDA’s Approval of Wezlana (Biosimilar, ustekinumab) for the Treatment of Multiple Inflammatory Diseases
Wezlana
Active ingredient: ustekinumab Approved: Oct 31, 2023
Company: Amgen Disease: multiple inflammatory disease
-
The approval was granted based on scientific evidence showing high similarity to Stelara with no clinically meaningful differences in safety and effectiveness and met the criteria to be interchangeable with Stelara
-
Wezlana has been approved for the adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderately to severely active Crohn's disease and moderately to severely active ulcerative colitis
-
It has also been approved for pediatric patients aged 6 yrs. to treat moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis
Related Post: Insights+: The US FDA New Drug Approvals in September 2023
Tags

Shivani was a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She was covering news related to Product approvals, clinical trial results, and updates. We can be contacted at connect@pharmashots.com.














